G
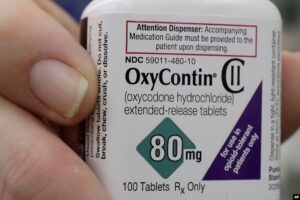
lobal consulting firm McKinsey & Co. has agreed to pay $650 million to settle a Department of Justice investigation into the firm’s consulting work with opioids manufacturer Purdue Pharma. The DoJ had accused McKinsey of fueling the opioid epidemic with its aggressive sales and marketing advice to Purdue, including a 2013 engagement in which McKinsey advised on steps to “turbocharge” sales of OxyContin.
McKinsey has agreed to pay a penalty of over $231 million, a forfeiture amount of over $93 million and a payment of $2 million to the Virginia Medicaid Fraud Control Unit to resolve the criminal allegations.
The settlement marks the first time a management consulting firm has been held criminally responsible for advice resulting in the commission of a crime by a client and reflects the Justice Department’s ongoing efforts to hold those responsible for their roles in the opioid crisis to account. The resolution is also the largest civil recovery for such conduct.
“For the first time in history, the Justice Department is holding a management consulting firm and one of its senior executives criminally responsible for the sales and marketing advice it gave resulting in the commission of crime by a client,” said Attorney Christopher Kavanaugh for the Western District of Virginia. “This ground-breaking resolution demonstrates the Justice Department’s ongoing commitment to hold accountable those companies and individuals who profited from our Nation’s opioid crisis.”
McKinsey also has entered into a civil settlement agreement in which it will pay over $323 million to resolve its liability under the False Claims Act for allegedly providing advice to Purdue that caused the submission of false and fraudulent claims to federal healthcare programs for medically unnecessary prescriptions of OxyContin, as well as allegedly failing to disclose to the U.S. Food and Drug Administration conflicts of interest arising from McKinsey’s concurrent work for Purdue and the FDA. This brings the total payments under the global resolution to $650 million.
McKinsey’s Criminal Liability for Misbranding
The criminal misbranding charge was based on McKinsey’s advice to the titan opioids manufacturer. Between 2004 and 2019, McKinsey contracted with Purdue on 75 different occasions in the United States. In 2007, a Purdue affiliate pleaded guilty to misbranding OxyContin, from 1996 through 2001, by falsely marketing it as less addictive, less subject to abuse and diversion, and less likely to cause dependence and withdrawal than other pain medications, and Purdue entered into a five-year corporate integrity agreement with the Department of Health and Human Services Office of Inspector General. After the 2007 guilty plea, McKinsey partners maintained close contact with Purdue, and in 2009, worked with Purdue to enhance “brand loyalty” for OxyContin and protect market share.
In 2010 McKinsey worked with Purdue to obtain FDA approval for a version of OxyContin that was reformulated with abuse-deterrent properties. Following the introduction of reformulated OxyContin in August 2010, OxyContin sales immediately began to decline. Purdue studied the drivers for this decline and attributed it, in large part, to a drop in prescriptions for individuals abusing OxyContin and increases in regulatory safeguards intended to hinder medically unnecessary prescribing of OxyContin.
In May 2013, Purdue retained McKinsey to conduct a rapid assessment of the underlying drivers of OxyContin performance, identify key opportunities to increase near-term OxyContin revenue and develop plans to capture priority opportunities. This 2013 effort was called Evolve to Excellence, or “E2E,” and included McKinsey advising Purdue on how to “turbocharge” the sales pipeline for OxyContin by, among other strategies, intensifying marketing to High Value Prescribers, included prescribers who were writing opioid prescriptions for uses that were unsafe, ineffective, and medically unnecessary.
McKinsey consultants spoke with Purdue about the concerns and increasing reluctance of pharmacists and pharmacy chains to fill prescriptions for OxyContin as abuse of the drug rose. McKinsey consultants also went on several “ride-alongs” with Purdue sales representatives in the field, as these sales representatives called on prescribers and pharmacists. In notes about one of these ride-alongs, a McKinsey consultant wrote, in part, “Pharmacist; [had] a gun and was shaking; abuse is definitely a huge issue[.]”
In August 2013, McKinsey partners met with certain members of the Purdue Board of Directors to present McKinsey’s findings and proposal; as one McKinsey partner reported afterwards, “[b]y the end of the meeting the findings were crystal clear to everyone and they gave a ringing endorsement of ‘moving forward fast.’” McKinsey also described for Purdue the financial value at stake: “hundreds of millions, not tens of millions.”
For Purdue and McKinsey, E2E was a financial success. Their targeting of High Value Prescribers slowed OxyContin’s declining sales and kept Purdue’s profits flowing at the expense of public health. After the conclusion of McKinsey’s work for Purdue on E2E, McKinsey performed additional work with Purdue that also sought to maximize OxyContin sales by further targeting sales efforts to High Value Prescribers.
False Claims to Federal Healthcare Programs and the FDA
The department’s civil False Claims Act settlement relates to allegations that, from 2013 to 2014, McKinsey, by advising Purdue to turbocharge OxyContin marketing to High Value Prescribers as a means to increase OxyContin sales, and despite its awareness of the opioid crises, knowingly caused false and fraudulent claims for OxyContin to be submitted to Medicare, Medicaid, TRICARE, the Federal Employees Health Benefit Program and the Veterans Health Administration.
The large $650 million fine also settles allegations that, from 2014 to 2017, McKinsey knowingly misled the FDA by assigning consultants to concurrently work on both FDA projects and competitively sensitive Purdue projects, contrary to McKinsey US’ conflict of interest policy. While soliciting a contract from the FDA, McKinsey US represented to the FDA that it had a conflict-of-interest policy in which its consultants serving the FDA would not be assigned to a competitively sensitive project for a significant period of time following an assignment for FDA.
The FDA then awarded McKinsey US the first in a series of contracts on a project relating to the monitoring of the safety of FDA-regulated products. McKinsey US admitted that it did not inform the FDA that its consultants worked on the Purdue projects around the same time those consultants also worked on the FDA project.
McKinsey’s Remedial Measures
As part of the resolution, McKinsey has agreed to implement a significant compliance program, including a system of policies and procedures designed to identify and assess high-risk client engagements. As part of this compliance program, McKinsey will implement new document retention procedures and training for all partners, officers and employees who provide or implement advice to clients. This compliance program is in addition to the provisions negotiated between McKinsey and the DoJ in a concurrent resolution with McKinsey & Company Africa that was announced on December 5th.
McKinsey has also agreed that it will not do any work related to the marketing, sale, promotion or distribution of controlled substances during the five-year term of the DPA. The settlement requires McKinsey’s Managing Partner to certify, on an annual basis, the firm’s compliance with its obligations under the DPA and federal law. ![]()
Jacob Horowitz is a contributing editor at Compliance Chief 360°

